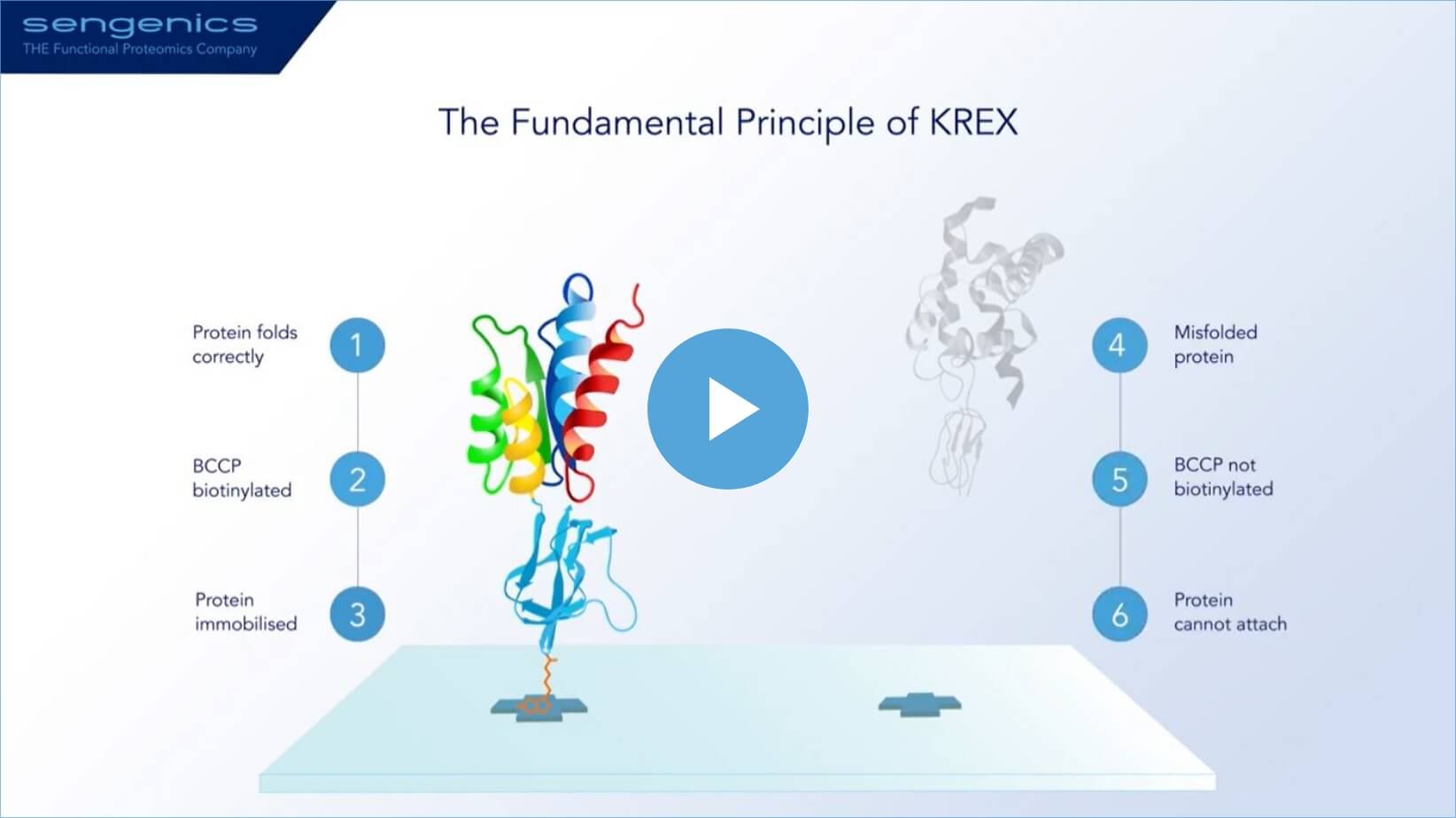
Based on an innovative principle
- KREX ensures that only correctly folded proteins are immobilized on the array surface.
- Misfolded proteins are prevented from attaching and are washed away.
- Immobilized proteins retain their folded structure, behaving as if they are in free solution on the hydrogel-coated array surface.
- This ensures each assay is run on correctly folded, full-length, functional proteins—crucial for autoantibody-based assays where more than 90% of antibodies recognize conformational epitopes that are only available if the protein is correctly folded.